Verhoeff van Gieson
Classification: connective tissue stain
Mechanism of staining: ionic bonding/van de Waals forces for elastin fibers
Purpose: stain elastin
Control tissue: aorta, skin, lung
Well Stained Slide
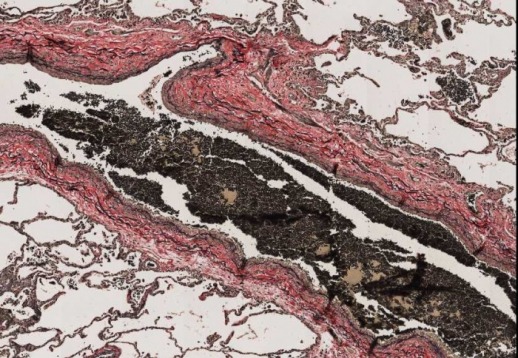
Elastic fibers – blue-black to black
Nuclei – blue/grey/black
Collagen – red
Muscle – orange
RBCs, cytoplasm – yellow
|
REAGENT |
PURPOSE |
MECHANISM OF STAINING |
SOURCE OF ERROR |
|
Iron Hematoxylin (Verhoeff’s) |
Primary stain for elastin |
A basic staining lake which is used
progressively for staining elastin and nuclei |
Omitted: Elastin
fibers will not be demonstrated. |
|
Too short: Elastin
will not be demonstrated. Differentiating time with ferric choloride will
need to be shorter. |
|||
|
Too long: Background
staining. Can remove the excess hematoxylin by leaving the slide in ferric
choloride for a longer time. |
|||
|
Ferric choloride (2%) |
Differentiator |
Removes brown discoloration |
Omitted: Overstained
slide. No details will be demonstrated. |
|
Too short: Under
differentiated. |
|||
|
Too long: Over
differentiated. |
|||
|
Sodium thiosulphate (5%) |
Omits iodine
discoloration by reacting to produce water-soluble sodium tetrathionate Removes mercury
pigments |
Forms complexes at the aldehyde groups |
Omitted: Van Geisson stain will be dull. |
|
Too short: Van Geisoon stain will be dull. |
|||
|
Too long: No effect. |
|||
|
Van Geisson |
Counterstain to visualize collagen, cytoplasm and muscles (contains two acid dyes such as picric acid and acid fuchsin) |
Ionic bonding
& porosity |
Omitted: Tissue
components will not be demonstrated. |
|
Too long: Counterstain
will obscure the primary stain and fine elastin fibers will be removed. |
|||
|
Too short: Background
tissues will stain less intensely. |
Special Considerations
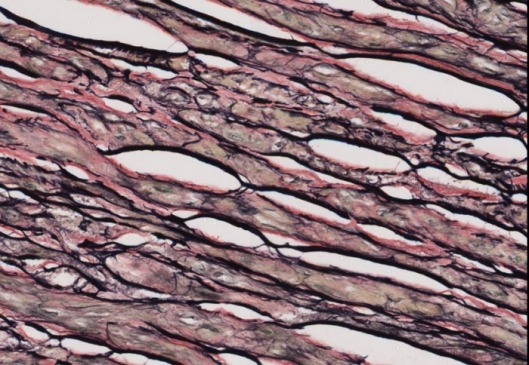
Purpose of VVG: Demonstration of pathologic elastin fibers, atrophy of elastic fibers, arterioclerosis or other vascular disorders, breaking of elastic fibers due to ageing process and demonstration of elastic fibers in normal tissues (veins and arteries)
Proper preparation of Verhoeff’s Iron hematoxylin solution is critical for successful staining. Usually 20ml of alcoholic Hematoxylin is mixed with 8ml of Ferric chloride(10%) and 8ml of lugols Iodine. This compound should be prepared prior to use and the ingredients must be added in order because if iodine is added first, the hematoxylin immediately would be overoxidized and a substance without staining properties would be made.The counterstain (Van Gesson) is usually used with pH between 1.0 & 2.0 which increases the number of dye binding sites for collagen. When doing the dehydration, it should be done rapidly so that the picric acid will not be lost from cytoplasm.
There is no need to remove mercury pigments by a separate procedures because Iodine will automatically remove these pigments during the staining method.
Clean glassware and staining tray with 2% FeCl3
References
Officer B. HIML251 Lecture notes: Elastic Stains: Verhoeff’s van Gieson & Gomori’s Aldehyde Fuchsin, January 21, 2009
